Uncovering Windermere’s hidden depths using environmental DNA monitoring
1 December, 2023
By Lynsey R. Harper (1), Bernd Hänfling (2) and Lori Lawson Handley (3)
(1) The Freshwater Biological Association, lharper@fba.org.uk; (2) University of the Highlands and Islands; (3) University of Hull
We report on environmental DNA monitoring of Windermere, from initial ground-truthing efforts for fish to citizen science assessment of other vertebrates. Many coauthors contributed to the research spearheaded by Bernd Hänfling and Lori Lawson Handley, and the most recent eDNA survey was made possible by volunteers.
Edited by Rachel Stubbington, Nottingham Trent University
Rachel is both a Fellow of the Freshwater Biological Association and long-standing Editor of FBA articles. If you would like to submit an article for consideration for publication, please contact Rachel at: rachel.stubbington@ntu.ac.uk
Background
Windermere, Cumbria, is an iconic lake under pressure from nutrient inputs, faecal pollution, tourism and recreation, land use change, invasive non-native species, and climate change (Fig. 1; Maberly & Elliott 2012). The biology of Windermere is relatively unknown compared to its water chemistry, geology, and hydrology. The lake’s phytoplankton, zooplankton and fish have excellent long-term monitoring records (Thackeray et al. 2013), but information on other animals and plants is lacking.
Figure 1. Windermere as seen from the FBA head office. Photo credit: Trine Bregstein.
Animals and plants leave traces of DNA in their environment, for example, hair, scales, urine, faeces, carcasses. This environmental DNA (eDNA) can be captured and analysed to identify which species it belongs to, similar to scanning a barcode, offering a non-invasive, cost-effective and comprehensive tool for biological surveys (Takahashi et al. 2023).
eDNA sampled from a lake reflects the species living in it as well as its catchment. eDNA can be deposited in lakes via river flow, surface water and groundwater runoff, and terrestrial animals directly interacting with the lake, for example, while drinking or urinating. Consequently, eDNA analysis of lake water samples can identify animal and plant life both in water and on land (Littlefair et al. 2023).
Laying the groundwork
Previous studies on Windermere from 2015–2016 showed that eDNA analysis detects more fish species than invasive and laborious methods such as gill-netting (Hänfling et al. 2016; Lawson Handley et al. 2019). In a single eDNA survey, 14 of the 16 fish species ever recorded in Windermere were detected (Fig. 2), compared to the 4–5 species that are typically caught by netting (Hänfling et al. 2016).
Figure 2. Proportion of samples in which individual fish species were detected using eDNA analysis in Windermere’s North and South Basins from 2015–2016. Species are ordered by long-term abundance in the North Basin. Reproduced from Lawson Handley (2020) with permission.
The spatial distribution of eDNA also reflected characteristics of Windermere’s North and South Basins, with species that favour less nutrient-rich conditions (e.g. Arctic charr, Salvelinus alpinus) detected only in the North Basin, and nutrient-tolerant species being more common in the nutrient-enriched South Basin (e.g. bream, Abramis brama; Lawson Handley et al. 2019). Additionally, follow-up work used eDNA analysis to effectively detect Arctic charr spawning locations and activity (Di Muri et al. 2022).
Blending with citizen science
Recently, Windermere has been in the spotlight due to potentially harmful algal blooms, which are likely to increase in frequency and intensity due to climate change. Blooms can limit dissolved oxygen availability, and toxin production can impact human and animal health, sometimes killing pets, livestock and wildlife (McGowan 2023).
In 2022, the Freshwater Biological Association and Lancaster University jointly launched the Big Windermere Survey, a citizen science project monitoring water quality in Windermere and the wider Leven catchment. Volunteers are trained to collect water samples from approximately 100 different locations on Windermere and connected rivers and lakes (Fig. 3). Samples are analysed for nutrient and bacterial concentrations that indicate the potential for algal blooms and faecal pollution, producing the largest, one-day snapshot of conditions in Windermere.
Figure 3. Big Windermere Survey volunteer collecting a water sample. Photo credit: Ian Wood.
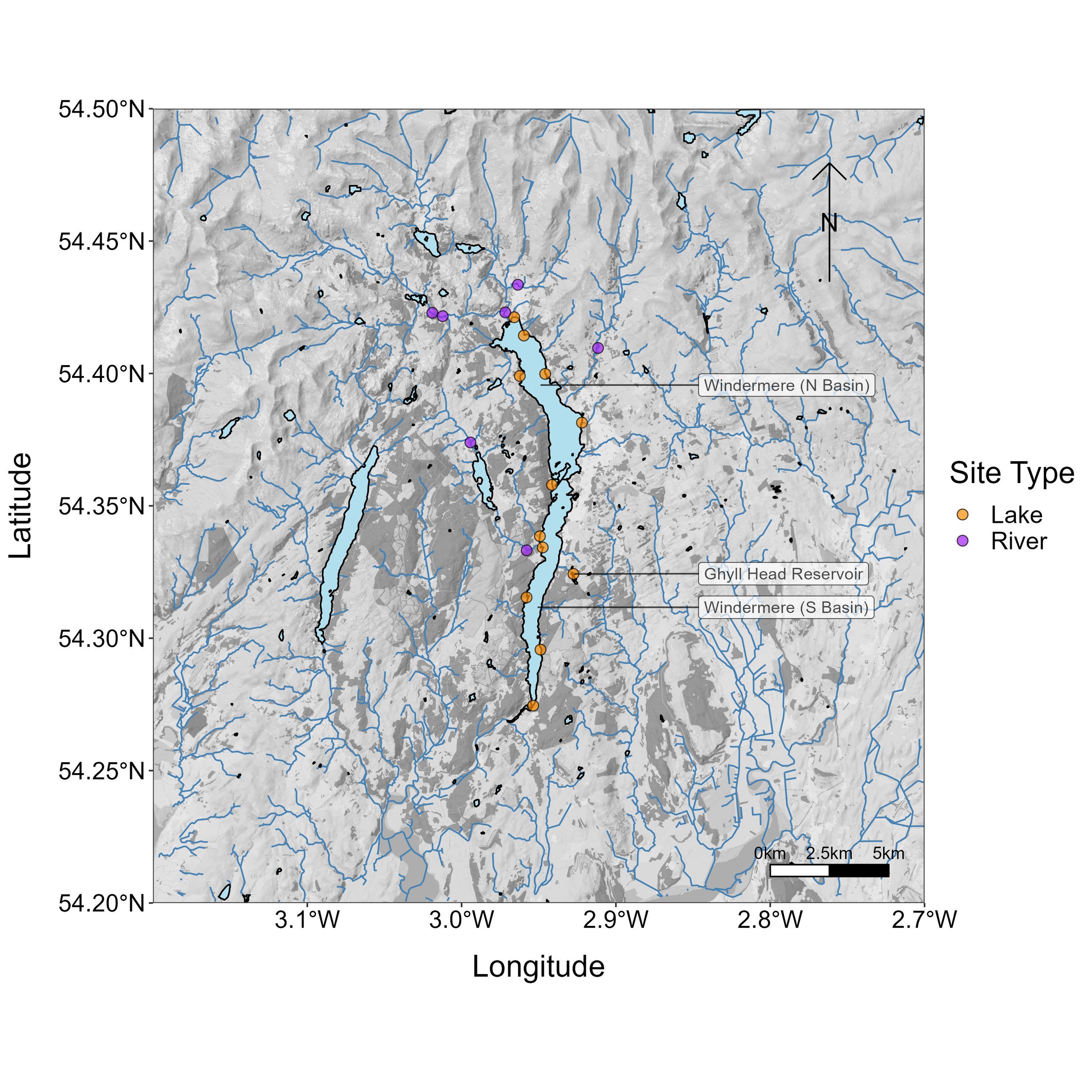
During the third Big Windermere Survey (5th February 2023), a pilot study took place to test whether citizen science eDNA monitoring can provide robust biological information for Windermere and its connected waterbodies. Volunteers collected 19 water samples (11 from Windermere, seven from connecting rivers, and one from Ghyll Head Reservoir; Fig. 4), which were filtered at the Freshwater Biological Association and analysed for vertebrates at the University of Hull.
Figure 4. Map of sampling locations for the Big Windermere Survey eDNA pilot.
Results
Across all 19 samples, 13 fish, three amphibians, 21 birds and 15 mammals were identified (Fig. 5). Common fish included minnow (Phoxinus phoxinus) and brown trout (Salmo trutta), and rare and protected species such as bullhead (Cottus gobio), eel (Anguilla anguilla) and lamprey (Lampetra spp.) were also detected.
Figure 5. Word cloud showing taxa detected using eDNA analysis, where word size is proportional to frequency of detection.
Considering the 11 Windermere samples, the most common fish were bullhead (10 samples), perch (Percidae) and minnow (8), bream and salmon (Salmo salar, 7), and roach (Rutilus rutilus, 6). In previous eDNA studies of Windermere (Hänfling et al. 2016; Lawson Handley et al. 2019), perch and roach dominated samples from both basins. Changes to sampling intensity and water collection and filtration methods may explain these differences.
The non-native rainbow trout (Oncorhynchus mykiss) was the only new species recorded in Windermere by the Big Windermere Survey eDNA pilot. The species was detected at Ghyll Head Reservoir where it is known to occur, and at low DNA concentration in one Windermere sample (North Basin, East shore). Arctic charr was not detected in the Big Windermere Survey eDNA pilot, likely because strategic sampling is needed for this fish, which prefers deeper, offshore waters outside the spawning season (Di Muri et al. 2022). Importantly, absence of Arctic charr DNA does not mean that they are absent from Windermere.
Common toad (Bufo bufo) and common frog (Rana temporaria) were detected in Windermere and connected rivers, while palmate newt (Lissotriton helveticus) was detected only in the River Brathay.
Common mammals included field vole (Microtus agrestis) and red deer (Cervus elaphus). House mouse (Mus musculus), common shrew (Sorex araneus), water shrew (Neomys fodiens) and badger (Meles meles) were each detected in one sample. Dog and sheep were often detected, while pig was detected in just five samples. Cattle and human were found in every sample, but also in the field negative control and so require careful interpretation.
Waterfowl were found in most samples at high DNA concentrations, particularly in Windermere. Pheasant (Phasianus colchicus), pigeons, and corvids were common, and songbirds (e.g. tits and finches) were also detected. Additional analyses may be needed to distinguish ducks, geese and swans for a full bird inventory.
Conclusions
Previous research and the Big Windermere Survey eDNA pilot highlight the range of wildlife supported by Windermere and the wider Leven catchment. eDNA analysis can be used to monitor fish as well as semi-aquatic and terrestrial animals, which could benefit local conservation projects (e.g. for hedgehog Erinaceus europaeus, otter Lutra lutra) and reintroduction programmes (e.g. for water vole Arvicola amphibius, pine marten Martes martes, red squirrel Sciurus vulgaris, beaver Castor fiber). Further eDNA surveys could help establish wildlife trends for Windermere and improve understanding of lake health.
Citizen scientist involvement not only extends eDNA monitoring across space and time, but connects volunteers with nature, increases awareness of local wildlife, and provides both learning and social opportunities (Broadhurst et al. 2023). The power of citizen science eDNA monitoring is already being harnessed in the UK through, for example, PondNet eDNA Great Crested Newt survey and GenePools. Combined with the chemical and bacteriological aspects of the Big Windermere Survey, eDNA monitoring offers volunteers a voice and enables them to communicate Windermere’s biology. Scaling up and permanently incorporating eDNA analysis into the Big Windermere Survey is hindered by cost, but would enhance understanding of the biological aspects of water quality in Windermere and the wider Leven catchment as well as nature connectedness.
Acknowledgements
The Big Windermere Survey eDNA pilot was funded by the Environment Agency and made possible by volunteers.
References
Broadhurst, H. et al. 2023. Citizen scientists’ motivation to participate in environmental DNA (eDNA) surveys: A case study on monitoring mammals in the UK. SocArXiv. https://doi.org/10.31235/osf.io/fa83k
Di Muri, C. et al. 2022. Spatio‐temporal monitoring of lake fish spawning activity using environmental DNA metabarcoding. Environmental DNA 5: 849–860. https://doi.org/10.1002/edn3.343
Hänfling, B. et al. 2016. Environmental DNA metabarcoding of lake fish communities reflects long-term data from established survey methods. Molecular Ecology 25: 3101–3119. https://doi.org/10.1111/mec.13660
Lawson Handley, L. et al. 2019. Temporal and spatial variation in distribution of fish environmental DNA in England’s largest lake. Environmental DNA 1: 26–39. https://doi.org/10.1002/edn3.5
Lawson Handley, L. 2020. From one lake to one thousand rivers: 21st century fish monitoring with environmental DNA. Salmo Trutta 23: 24–29. https://www.wildtrout.org/salmo/
Littlefair, J.E. et al. 2023. Freshwater connectivity transforms spatially integrated signals of biodiversity. Proceedings of the Royal Society B: Biological Sciences 290: 20230841. https://doi.org/10.1098/rspb.2023.0841
Maberly, S.C. & Elliott, J.A. 2012. Insights from long-term studies in the Windermere catchment: external stressors, internal interactions and the structure and function of lake ecosystems. Freshwater Biology 57: 233–243. https://doi.org/10.1111/j.1365-2427.2011.02718.x
McGowan, S. 2023. Chapter 2 - Harmful algal blooms. Biological and Environmental Hazards, Risks, and Disasters (Second Edition) (eds R. Sivanpillai & J.F. Shroder), pp. 9–53. Elsevier, Boston. https://doi.org/10.1016/B978-0-12-820509-9.00002-2
Takahashi, M. et al. 2023. Aquatic environmental DNA: A review of the macro-organismal biomonitoring revolution. Science of the Total Environment 873: 162322. https://doi.org/10.1016/j.scitotenv.2023.162322
Thackeray, S.J. et al. 2013. Food web de-synchronization in England’s largest lake: an assessment based on multiple phenological metrics. Global Change Biology 19: 3568–3580. https://doi.org/10.1111/gcb.12326
Further reading
The Freshwater Biological Association publishes a wide range of books and offers a number of courses throughout the year. Check out our shop here.
Get involved
Our scientific research builds a community of action, bringing people and organisations together to deliver the urgent action needed to protect freshwaters. Join us in protecting freshwater environments now and for the future.